About Bryxta
Bevacizumab (400 mg/ 100 mg)
Bryxta™ is a recombinant humanized monoclonal antibody (containing 1337 amino acids) produced in Chinese Hamster Ovary cell line. VEGF is a signal protein which stimulates vasculogenesis and angiogenesis. Bevacizumab binds to VEGF and inhibits its interactions with VEGF receptors (VEGFRs), Flt-1 (VEGFR-1) and KDR (VEGFR-2), on the surface of endothelial cells. This results in regression of tumour vasculature and inhibition of new tumour vessel growth.
- Indications
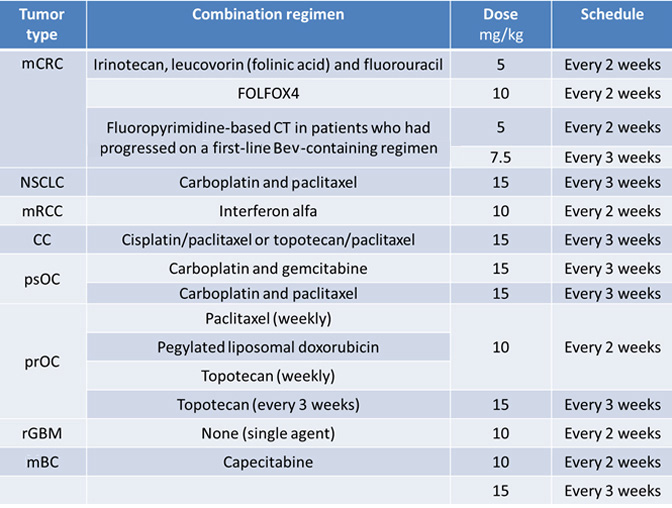
- Metastatic Colorectal Cancer
- Non-Squamous Non–Small Cell Lung Cancer
- Glioblastoma
- Metastatic Breast Cancer
- Metastatic Renal Cell Carcinoma
- Persistent, Recurrent, or Metastatic Carcinoma of the Cervix
- Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer
- Dosage and administration
- Preparation for administration
Bevacizumab should be diluted for infusion using aseptic technique. Withdraw the necessary amount to obtain the required dose and dilute in a total volume of 100 mL of 0.9% Sodium Chloride Inj. Inspect visually for particulate matter and discoloration prior to administration. Discard any unused portion left in a vial. Diluted solutions for infusion may be stored at 2°C–8°C (36°F–46°F) for up to 8 hours. Bevacizumab infusions should not be administered or mixed with dextrose solution.
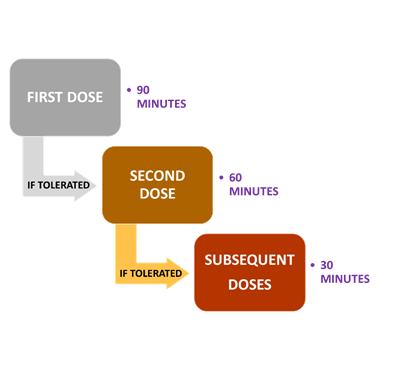
- Administration and infusion time
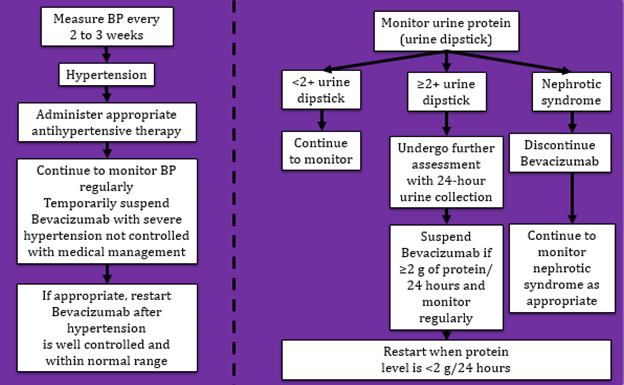
- Hypertension and proteinuria: Monitoring and management
- Special warnings & precautions
- Epistaxis
- Proteinuria
- Lacrimation Disorder
- Headache
- Taste Alteration
- Back Pain
- Hypertension
- Dry Skin
- Exfoliative Dermatitis
- Rhinitis
- Rectal Hemorrhage
- Management of adverse effects
When to discontinue
- GI perforations (GI perforations, fistula formation in the GI tract, intra-abdominal abscess)
- Fistula formation involving an internal organ
- Wound dehiscence and wound healing complications requiring medical intervention
- Serious hemorrhage (ie, requiring medical intervention)
- Severe arterial thromboembolic event (ATE)
- Life-threatening (grade 4) venous thromboembolic events, including pulmonary embolism
- Hypertensive crisis or hypertensive encephalopathy
- Posterior reversible encephalopathy syndrome (PRES)
- Nephrotic syndrome
When to interrupt
- At least 4 weeks prior to elective surgery
- Severe hypertension not controlled with medical management
- Moderate to severe proteinuria
- Severe infusion reactions
- Fertility, pregnancy and lactation

Women of childbearing potential
Women of childbearing potential have to use effective contraception during (and up to 6 months after) treatment.

Pregnancy
There are no clinical trial data on the use of bevacizumab in pregnant women. Bevacizumab may cause fetal harm based on findings from animal studies and the drug’s mechanism of action. In the post-marketing setting, cases of fetal abnormalities have been reported in women treated with bevacizumab alone or in combination with known embryotoxic chemotherapeutics. Bevacizumab is contraindicated in pregnancy.

Nursing Mothers
It is not known whether bevacizumab is excreted in human milk. As maternal IgG is excreted into human milk, and the potential for harm to the infant is unknown, women must discontinue breast-feeding during therapy and not breast-feed for at least six months following the last dose of Bevacizumab.

Fertility
Repeat dose toxicity studies in animals have shown that bevacizumab may have an adverse effect on female fertility long term effects of bevacizumab treatment on fertility are unknown.